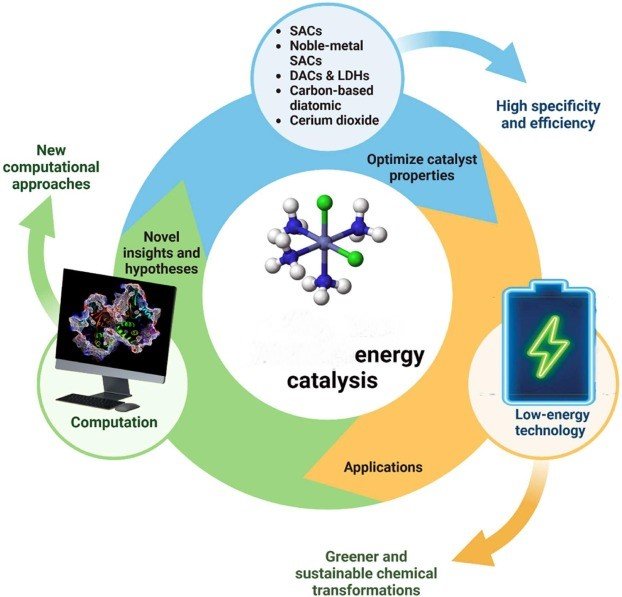
Catalysts are essential for improving the efficiency, sustainability, and economic viability of basic chemical processes. By accelerating chemical reactions without being consumed, catalysts enable processes to occur under milder conditions, with higher selectivity and reduced energy input. Their role spans across various aspects of chemical manufacturing, making them a cornerstone of modern industrial chemistry.
1. Enhancing Reaction Rates
- Lower Activation Energy:
- Catalysts reduce the energy barrier for chemical reactions, enabling reactions to occur more quickly and at lower temperatures.
- Impact:
- Shorter reaction times increase productivity and throughput, especially in high-demand industries like petrochemicals and fertilizers.
2. Reducing Energy Consumption
- Operating Under Milder Conditions:
- Many catalytic processes reduce the need for high temperatures and pressures, which are energy-intensive.
- Impact:
- Lower energy requirements decrease operational costs and carbon emissions, contributing to sustainability goals.
- Example:
- Zeolite catalysts in hydrocracking allow oil refining processes to operate efficiently at lower temperatures.
3. Improving Selectivity
- Targeted Product Formation:
- Catalysts improve selectivity by favoring specific reaction pathways, reducing the formation of unwanted by-products.
- Impact:
- Higher selectivity reduces waste, lowers purification costs, and increases overall yield.
- Example:
- Platinum-based catalysts in ammonia oxidation for nitric acid production ensure minimal side reactions.
4. Enabling Sustainable Processes
- Green Chemistry Applications:
- Catalysts are integral to sustainable chemical processes, including carbon capture, renewable feedstock utilization, and waste valorization.
- Impact:
- Facilitates the transition to cleaner and more environmentally friendly chemical production.
- Example:
- Iron-based catalysts in Fischer-Tropsch synthesis convert syngas into fuels and chemicals, using renewable or waste-derived carbon sources.
5. Supporting Energy Transition
- Hydrogen Economy:
- Catalysts are critical in producing, storing, and utilizing hydrogen, a key element in decarbonizing energy systems.
- Impact:
- Improves the efficiency of hydrogen production processes like water electrolysis and methane reforming.
- Example:
- Nickel catalysts are widely used in steam methane reforming (SMR) for hydrogen production.
6. Driving Innovation in Basic Chemical Production
- Ammonia Synthesis (Haber-Bosch Process):
- Iron-based catalysts are used to synthesize ammonia efficiently, a process critical for fertilizer production.
- Sulfuric Acid Production:
- Vanadium pentoxide (V₂O₅) catalysts improve the oxidation of sulfur dioxide (SO₂) to sulfur trioxide (SO₃), a key step in sulfuric acid production.
- Ethylene and Propylene Production:
- Zeolite catalysts enhance the cracking of hydrocarbons to produce olefins, essential building blocks in the chemical industry.
7. Reducing Environmental Impact
- Pollution Control:
- Catalysts are essential for reducing emissions, such as flue gas treatment and catalytic converters in vehicles.
- Impact:
- Minimizes the environmental footprint of industrial processes.
- Example:
- Palladium and rhodium catalysts in catalytic converters remove harmful emissions like carbon monoxide (CO) and nitrogen oxides (NOₓ).
8. Enabling Circular Economy
- Recycling and Upcycling:
- Catalysts facilitate the conversion of waste materials into valuable chemicals or fuels.
- Impact:
- Supports resource efficiency and reduces reliance on virgin raw materials.
- Example:
- Metal-organic frameworks (MOFs) are being developed for chemical recycling of plastics.
9. Supporting Process Intensification
- Compact and Integrated Systems:
- Catalysts allow for process intensification, combining multiple reaction steps into a single reactor.
- Impact:
- Reduces plant size, energy usage, and capital investment.
- Example:
- Multifunctional catalysts for single-step conversion of syngas to methanol or dimethyl ether.
10. Innovations in Catalysis
- Heterogeneous Catalysts:
- Solid catalysts that operate in a different phase from reactants are widely used for ease of separation and reuse.
- Homogeneous Catalysts:
- Offer high selectivity for complex reactions, though separation can be challenging.
- Biocatalysts:
- Enzymes provide highly selective and energy-efficient alternatives for industrial chemical synthesis.
- Nanocatalysts:
- Nanostructured catalysts offer enhanced surface area and activity, driving innovations in efficiency and selectivity.
- Electrocatalysts:
- Crucial for processes like water splitting and CO₂ reduction in renewable energy systems.
Conclusion
Catalysts are at the heart of modern chemical manufacturing, enabling efficient, sustainable, and innovative processes. By reducing energy consumption, improving selectivity, and minimizing environmental impact, catalysts drive the chemical industry’s progress toward a greener and more economically viable future. Ongoing advancements in catalysis technology will further enhance their role in addressing global challenges like climate change, resource scarcity, and energy transition.
Hashtags
#Catalysis #ChemicalCatalysts #CatalystInnovation #CatalysisInChemistry #IndustrialCatalysts #Efficiency and Process Optimization #ProcessEfficiency #OptimizedChemistry #ChemicalProcessImprovement #EfficiencyThroughCatalysts #SmartChemistry #Sustainability and Green Chemistry #GreenCatalysis #SustainableProcesses #EcoFriendlyChemistry #CatalystsForSustainability #LowCarbonChemistry #Advanced Technology and Innovation #CatalystTech #InnovativeCatalysts #NanoCatalysts #AdvancedMaterials #TechInChemistry