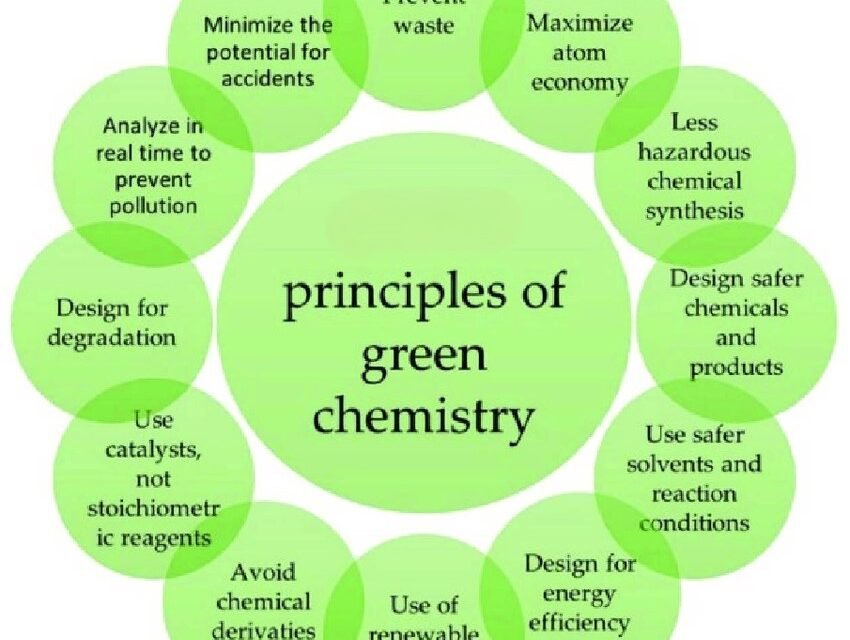
The principles of green chemistry provide a framework for designing chemical processes and products that reduce or eliminate the use and generation of hazardous substances. Developed by Paul Anastas and John Warner in the 1990s, these 12 principles promote sustainability, efficiency, and environmental protection in chemistry. Here’s an overview of the principles:
1. Prevention
- Goal: Avoid generating waste rather than treating or cleaning it up after it is created.
- Example: Designing chemical processes with higher atom efficiency to minimize byproducts.
2. Atom Economy
- Goal: Maximize the incorporation of all starting materials (atoms) into the final product.
- Calculation: Atom economy is expressed as a percentage of the total mass of desired products relative to all reactants.
- Example: Using catalytic reactions instead of stoichiometric ones to reduce waste.
3. Less Hazardous Chemical Syntheses
- Goal: Design synthetic methods that use and generate substances with minimal toxicity to humans and the environment.
- Example: Using hydrogen peroxide as an oxidant instead of hazardous chromium-based oxidizing agents.
4. Designing Safer Chemicals
- Goal: Create chemical products that are effective but have reduced toxicity.
- Example: Developing pesticides that are toxic to target organisms but harmless to non-target species.
5. Safer Solvents and Auxiliaries
- Goal: Minimize or eliminate the use of auxiliary substances like solvents or separation agents.
- Example: Replacing organic solvents with water or supercritical CO₂ in chemical processes.
6. Design for Energy Efficiency
- Goal: Minimize energy use by conducting reactions at ambient temperature and pressure whenever possible.
- Example: Using microwave or ultrasound technologies to reduce reaction times and energy consumption.
7. Use of Renewable Feedstocks
- Goal: Prefer raw materials that are renewable rather than depletable.
- Example: Sourcing feedstocks from agricultural or waste products, such as bioethanol from sugarcane.
8. Reduce Derivatives
- Goal: Avoid unnecessary steps like using blocking or protecting groups, which generate waste.
- Example: Directly functionalizing a molecule without intermediate modifications.
9. Catalysis
- Goal: Use catalysts (which are not consumed in the reaction) instead of stoichiometric reagents.
- Example: Employing metal catalysts in hydrogenation reactions to reduce waste and improve efficiency.
10. Design for Degradation
- Goal: Design products that break down into benign substances after use.
- Example: Developing biodegradable plastics that decompose into water and carbon dioxide.
11. Real-Time Analysis for Pollution Prevention
- Goal: Monitor reactions in real time to detect and minimize the formation of hazardous byproducts.
- Example: Using in-line spectroscopy for immediate feedback during manufacturing processes.
12. Inherently Safer Chemistry for Accident Prevention
- Goal: Use substances and processes that minimize the risk of chemical accidents, such as explosions or toxic releases.
- Example: Avoiding highly reactive or unstable reagents by choosing safer alternatives.
Key Impacts of Green Chemistry
- Environmental Benefits:
- Reduces pollution and waste generation.
- Encourages the use of renewable and biodegradable resources.
- Economic Advantages:
- Increases efficiency and reduces costs by minimizing waste and energy consumption.
- Health and Safety:
- Reduces exposure to toxic substances and risks of chemical accidents.
- Innovation:
- Drives the development of new, sustainable technologies and products.
Conclusion
The principles of green chemistry represent a shift toward a more sustainable, safe, and economically viable chemical industry. By integrating these principles, industries can create processes and products that benefit both society and the environment, paving the way for a sustainable future in chemistry and manufacturing.
Hashtags
#GreenChemistry #PrinciplesOfGreenChemistry #SustainableChemistry #EcoChemistry #ChemistryForGood #AtomEconomy #WastePrevention #RenewableFeedstocks #GreenCatalysis #EnergyEfficiencyInChemistry #DesignForSustainability #DesignForEnvironment #SafeChemicals #NonToxicChemicals #EnvironmentallyFriendlyChemicals #EfficientChemistry #SustainableInnovation #SmartChemistry #ChemicalInnovationForGood #ChemicalOptimization#GreenChemistryInPractice #SustainableManufacturing #GreenChemistryInAction #EcoFriendlyProcesses #SustainableChemicalProcesses #GreenTechChemicals