Polymers are large molecules (macromolecules) composed of repeating structural units, called monomers, that are covalently bonded together. They are the building blocks of many materials used in everyday life, including plastics, fibers, rubber, and adhesives. Polymers exhibit a wide range of properties and applications, depending on their chemical structure and arrangement.
Here’s an overview of polymers and their classification:
Definition of Polymers
- Polymer:
- Derived from the Greek words poly (many) and meros (parts).
- Consist of repeating monomer units linked through polymerization processes (e.g., addition, condensation).
- Can have linear, branched, or cross-linked structures.
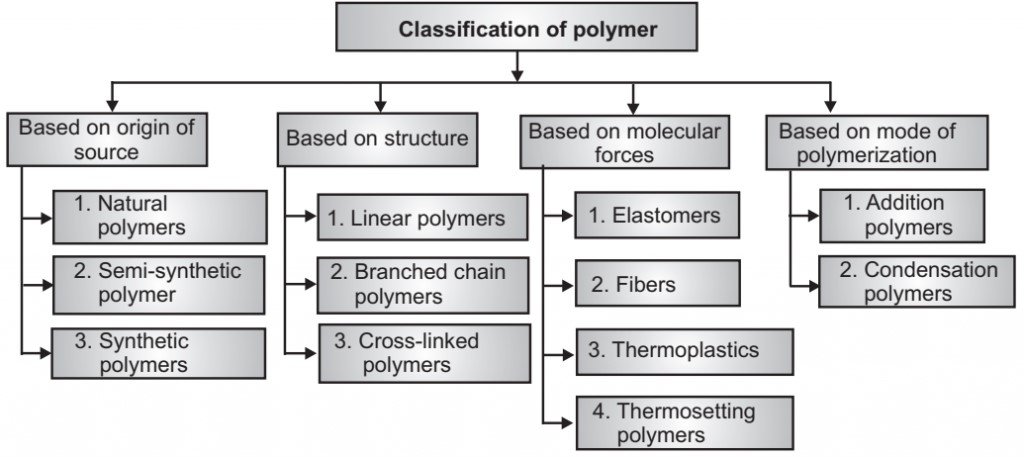
Classification of Polymers
Polymers can be classified based on several criteria, including origin, structure, polymerization method, properties, and applications.
1. Based on Origin
- Natural Polymers:
- Found in nature and produced by living organisms.
- Examples:
- Proteins (e.g., collagen, keratin).
- Polysaccharides (e.g., cellulose, starch, chitin).
- Natural rubber (polyisoprene).
- Applications: Food, textiles, medical, adhesives.
- Synthetic Polymers:
- Man-made polymers created through chemical synthesis.
- Examples:
- Polyethylene, polypropylene, polystyrene.
- Applications: Packaging, automotive parts, electronics.
- Semi-Synthetic Polymers:
- Derived from natural polymers but chemically modified.
- Examples:
- Cellulose acetate (used in films and textiles).
- Vulcanized rubber.
2. Based on Structure
- Linear Polymers:
- Monomers are connected in a straight chain.
- Properties: High density, crystalline structure, strong intermolecular forces.
- Examples:
- Polyethylene, polyvinyl chloride (PVC).
- Branched Polymers:
- Side chains branch off the main chain.
- Properties: Lower density and weaker intermolecular forces.
- Examples:
- Low-density polyethylene (LDPE).
- Cross-Linked Polymers:
- Chains are interconnected by covalent bonds.
- Properties: Rigid, durable, and heat-resistant.
- Examples:
- Bakelite, vulcanized rubber.
- Network Polymers:
- Extensive cross-linking creates a 3D network.
- Examples:
- Epoxy resins, polyurethane.
3. Based on Polymerization Method
- Addition Polymers:
- Formed by the repeated addition of monomers with double or triple bonds.
- No by-products are produced.
- Examples:
- Polyethylene, polypropylene, polystyrene.
- Condensation Polymers:
- Formed by the reaction of monomers with the elimination of small molecules (e.g., water, HCl).
- Examples:
- Nylon, polyester, polycarbonate.
4. Based on Thermal Behavior
- Thermoplastics:
- Can be repeatedly melted and reshaped upon heating.
- Properties: Flexible, recyclable, low melting points.
- Examples:
- Polyethylene (PE), polystyrene (PS), polyvinyl chloride (PVC).
- Applications: Packaging, bottles, pipes.
- Thermosetting Polymers:
- Harden permanently upon heating and cannot be reshaped.
- Properties: Rigid, strong, heat-resistant.
- Examples:
- Bakelite, epoxy resin, vulcanized rubber.
- Applications: Electrical insulators, adhesives, coatings.
5. Based on Physical Properties
- Elastomers:
- Rubber-like materials with elastic properties.
- Can stretch significantly and return to original shape.
- Examples:
- Natural rubber, neoprene, silicone rubber.
- Applications: Tires, seals, gaskets.
- Fibers:
- Polymers with high tensile strength and low elasticity.
- Examples:
- Nylon, polyester, Kevlar.
- Applications: Textiles, ropes, protective gear.
- Plastics:
- Can be molded into different shapes.
- Rigid Plastics: Polycarbonate, polystyrene.
- Flexible Plastics: Polyethylene, polypropylene.
- Adhesives:
- Used for bonding materials.
- Examples:
- Epoxies, cyanoacrylate (superglue).
6. Based on Chain Composition
- Homopolymers:
- It is composed of a single type of monomer.
- Examples:
- Polyethylene (from ethylene), polystyrene (from styrene).
- Copolymers:
- Formed from two or more different monomers.
- Types:
- Random Copolymers: Monomers arranged randomly.
- Block Copolymers: Long sequences of each monomer type.
- Graft Copolymers: Side chains of one monomer grafted onto a leading chain of another.
- Examples:
- ABS (acrylonitrile-butadiene-styrene), styrene-butadiene rubber (SBR).
7. Based on Degradability
- Biodegradable Polymers:
- Break down naturally through microbial activity.
- Examples:
- Polylactic acid (PLA), polyhydroxyalkanoates (PHA).
- Applications: Packaging, medical sutures.
- Non-Biodegradable Polymers:
- Do not degrade quickly and persist in the environment.
- Examples:
- Polyethylene, polystyrene.
Applications of Polymers
- Packaging:
- Plastic bags, bottles, films (e.g., polyethylene, polypropylene).
- Construction:
- PVC pipes, insulation, flooring.
- Automotive:
- Rubber tires, dashboards, bumpers.
- Electronics:
- Insulators, connectors, circuit boards.
- Textiles:
- Nylon, polyester fabrics.
- Medical:
- Biodegradable sutures, prosthetics, drug delivery systems.
- Aerospace:
- Lightweight composites, adhesives, heat shields.
Conclusion
Polymers are versatile materials classified based on origin, structure, properties, and applications. From natural polymers like cellulose to advanced synthetic polymers like Kevlar, their wide range of properties makes them indispensable in modern life. With ongoing advancements, especially in bio-based and sustainable polymers, their role in addressing environmental challenges is expanding.
Hashtags
#Polymers #PolymerChemistry #PolymerMaterials #AdvancedPolymers #PolymerInnovation #Thermoplastics #ThermosettingPolymers #Elastomers #ConductivePolymers #BiodegradablePolymers #SyntheticPolymers #NaturalPolymers #BioBasedPolymers #PolymerFromRenewables #PetrochemicalPolymers#LinearPolymers #BranchedPolymers #CrosslinkedPolymers #AmorphousPolymers #CrystallinePolymers #EngineeringPolymers #MedicalPolymers #FoodPackagingPolymers #AerospacePolymers #PolymerInConstruction#BiodegradablePolymers #EcoFriendlyPolymers #GreenPolymers #SustainablePlastics