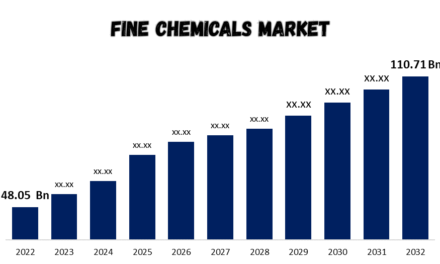
Polymers are synthesized in the chemical industry through various methods, depending on the type of polymer desired, the monomers involved, and the application requirements. Below are the key methods of polymer synthesis:
1. Addition Polymerization (Chain-Growth Polymerization)
This method involves the linking of monomers that contain a double bond (unsaturation) through a chain reaction. The polymerization process is initiated by a free radical, cation, or anion, depending on the type of polymerization.
- Process:
- Initiation: A free radical or other initiator creates an active center on a monomer, initiating the polymerization.
- Propagation: The active center reacts with other monomers, adding them to the growing polymer chain.
- Termination: The chain reaction stops, either by the combination of two active centers or by deactivating the active site.
- Common Polymers:
- Polyethylene (PE)
- Polystyrene (PS)
- Polypropylene (PP)
- Polyvinyl chloride (PVC)
2. Condensation Polymerization (Step-Growth Polymerization)
Condensation polymerization involves the reaction of two or more different monomers that contain functional groups (such as hydroxyl, amine, or carboxyl) to form a polymer, releasing small molecules like water or methanol as by-products.
- Process:
- Reaction of Monomers: Two monomers with complementary functional groups react, forming a bond and releasing a small molecule (e.g., water).
- Stepwise Growth: The reaction can continue as longer polymer chains are built by the stepwise reaction between monomers.
- Common Polymers:
- Polyester (e.g., PET)
- Nylons (e.g., Nylon-6, Nylon-66)
- Polyurethanes (e.g., used in foams, coatings)
3. Ring-Opening Polymerization (ROP)
Ring-opening polymerization is a type of addition polymerization where a cyclic monomer undergoes polymerization by opening its ring structure to form a linear polymer.
- Process:
- Initiation: A ring-shaped monomer is opened by an initiator (often a metal catalyst or organic compound).
- Propagation: The monomer adds to the growing chain in a repetitive manner, leading to the formation of a polymer.
- Common Polymers:
- Polycaprolactam (Nylon-6)
- Polylactic acid (PLA)
- Polyethylene glycol (PEG)
4. Emulsion Polymerization
Emulsion polymerization is a type of free-radical polymerization that occurs in an emulsion, usually a mixture of water, surfactants, and monomers. It’s widely used to create latexes and aqueous dispersions.
- Process:
- Emulsion Formation: The monomers are dispersed in water with surfactants, creating micelles (small particles).
- Polymerization: The polymerization occurs within the micelles, where the monomers react to form polymer chains.
- Common Polymers:
- Polyvinyl acetate (used in adhesives, paints)
- Acrylic polymers (used in coatings, adhesives)
5. Solvent Polymerization
Solvent polymerization is a method where monomers are dissolved in a solvent and polymerized using heat or catalysts. This method is used for producing high-quality polymers with specific properties.
- Process:
- Monomer Dissolution: Monomers are dissolved in a solvent to create a homogenous solution.
- Polymerization: The polymerization is initiated by heat, light, or a catalyst to form the polymer in the solvent.
- Common Polymers:
- Polystyrene (PS)
- Polyvinyl chloride (PVC)
6. Co-Polymerization
Co-polymerization involves the polymerization of two or more different monomers to form a copolymer with distinct properties that can be tailored for specific applications.
- Process: Two different monomers are polymerized simultaneously, and their arrangement in the final polymer chain can vary (e.g., alternating, block, or random arrangement).
- Common Polymers:
- Styrene-butadiene rubber (SBR)
- Acrylic-styrene copolymers
7. Ionic Polymerization
Ionic polymerization involves the use of ionic initiators (either cations or anions) to start the polymerization of monomers. This method is typically used to synthesize polymers with controlled molecular weight and structure.
- Process: The ionic initiator generates a charged center that reacts with the monomer, initiating polymerization.
- Common Polymers:
- Polystyrene
- Polybutadiene
8. Photopolymerization
Photopolymerization is a polymerization process that is initiated by exposure to light, usually ultraviolet (UV) radiation. This process is used in the production of coatings, inks, and certain 3D printing applications.
- Process:
- Initiation: Light activates a photoinitiator, which then starts the polymerization process.
- Polymerization: The monomers undergo rapid polymerization, often at room temperature.
- Common Polymers:
- Acrylic-based polymers (used in coatings)
- Epoxy resins (used in 3D printing and adhesives)
Each synthesis method offers specific advantages and is chosen based on the desired characteristics of the final polymer product, including strength, flexibility, thermal stability, and processing ease.