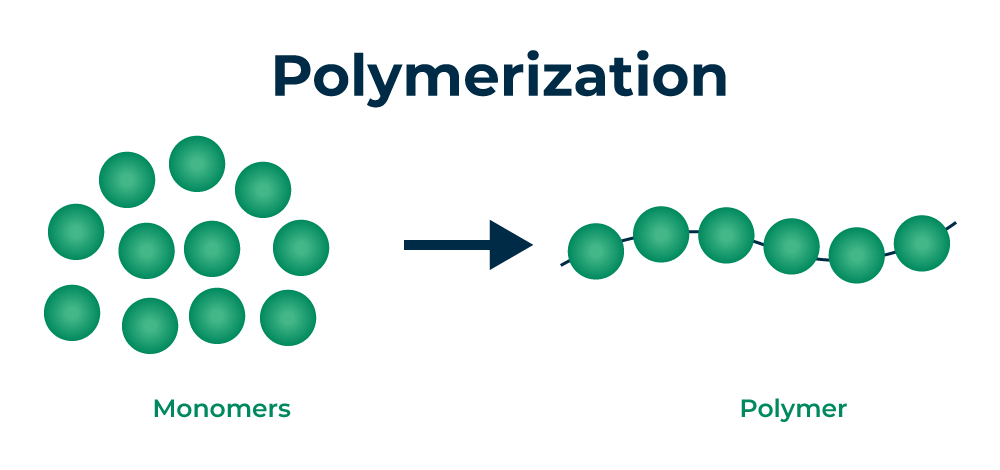
Polymerization is the chemical process through which monomers (small molecules) chemically bond together to form polymers (large molecules with repeating structural units). This process plays a crucial role in determining the properties of the final polymer, such as its strength, flexibility, thermal stability, density, and durability.
Types of Polymerization
There are two main types of polymerization:
1. Addition Polymerization (Chain-Growth Polymerization)
In addition polymerization, the monomers contain a double bond (unsaturation), which is broken and used to link together into a polymer chain. This type of polymerization is initiated by a free radical, cation, or anion, and does not involve the loss of any small molecules.
Process Steps:
- Initiation: A free radical (or other initiator) reacts with a monomer, opening its double bond to create a reactive site.
- Propagation: The reactive site continues to add more monomers, forming a long polymer chain.
- Termination: The polymerization ends when two reactive chains combine or when the reactive site is deactivated.
Effects on Polymer Properties:
- Molecular weight: Addition polymerization can produce polymers with high molecular weights, which can affect the polymer’s strength and viscosity.
- Structure: The polymer chains formed are typically linear or branched, impacting the density and crystallinity of the final polymer. For example, polyethylene (PE) can be high-density (HDPE) or low-density (LDPE) based on how the polymer chains arrange themselves.
- Flexibility and Strength: The extent of polymerization (chain length) influences the flexibility and strength of the polymer. Longer chains tend to make the material more robust.
2. Condensation Polymerization (Step-Growth Polymerization)
In condensation polymerization, two or more different monomers with complementary functional groups react, often releasing small molecules such as water or methanol in the process. This method is used to create polyesters, polyamides, and polyurethanes.
Process Steps:
- Reaction: Monomers with reactive groups (such as hydroxyl and carboxyl) react with each other, forming bonds and releasing small molecules.
- Stepwise Growth: The reaction proceeds step by step, with each new monomer adding to the growing polymer chain.
Effects on Polymer Properties:
- Molecular Weight: Condensation polymerization typically produces polymers with lower molecular weights compared to addition polymerization. However, by controlling reaction conditions, high-molecular-weight polymers can be achieved.
- Crystallinity: Condensation polymers tend to have higher crystallinity, which can increase their strength and thermal resistance.
- Functional Groups: The types of functional groups involved (e.g., amides, esters) determine the polymer’s chemical resistance, solubility, and thermal properties. For example, Nylon (a polyamide) is strong and resistant to wear, while polyethylene terephthalate (PET) is durable and commonly used in fabrics and bottles.
Factors Affecting Polymer Properties
The polymerization process directly influences several key properties of the final polymer:
- Molecular Weight
- Higher molecular weight results in stronger and more durable polymers, while lower molecular weight tends to lead to more flexible and easier-to-process materials.
- Polymer Structure
- The way monomers are connected affects the polymer’s crystallinity, density, and intermolecular forces. For example, linear polymers generally have higher crystallinity and better mechanical properties than branched polymers.
- Chain Arrangement
- Whether the polymer chains are linear, branched, or cross-linked influences the final properties:
- Linear polymers: Often have good tensile strength and flexibility.
- Branched polymers: Tend to have lower density and lower strength but can offer more flexibility.
- Cross-linked polymers: Have enhanced thermal stability and chemical resistance but are more rigid and less flexible (e.g., epoxy resins and vulcanized rubber).
- Whether the polymer chains are linear, branched, or cross-linked influences the final properties:
- Degree of Polymerization
- This refers to the number of monomer units in a polymer chain. A higher degree of polymerization usually leads to better mechanical properties, such as strength and toughness.
- Additives and Catalysts
- Catalysts are used to control the polymerization rate and molecular weight, while additives (such as stabilizers, plasticizers, and fillers) can modify properties like flexibility, color, UV resistance, and flame retardancy.
- Temperature and Pressure
- The conditions under which polymerization occurs—such as temperature and pressure—can affect the molecular weight and structure of the polymer, thus influencing the final product’s properties.
Impact on Common Polymers
- Polyethylene (PE): A polymer produced by addition polymerization. The polymer’s density (HDPE vs. LDPE) depends on the polymerization conditions and chain branching.
- Polypropylene (PP): Similar to PE, but it has a higher melting temperature and is more rigid.
- Polyester (PET): A condensation polymer. Its properties, such as strength and crystallinity, depend on the polymerization conditions, influencing its applications in textiles and containers.
- Nylon: A condensation polymer used for fibers and plastics. The properties of nylon, such as strength and abrasion resistance, are influenced by its molecular weight and degree of crystallinity.